Siddharth Annaldasula
Supervisors: Kirsten Kübler

Rosario Astaburuaga
Understanding the signalling pathways governing cell fate decisions after radiation-induced DNA damage in HN cancer cells
supervisors: Nils Blüthgen, Kirsten Lauber, Hanspeter Herzel
Ionizing radiation is the main treatment strategy for head and neck (HN) cancers, as it can damage the DNA of cancer cells. As HN tumours are usually highly heterogeneous, after DNA damage some cells easily die (i.e, radio-sensitive cells), but others resist to die and survive (i.e., radio-resistant cells). The mechanisms governing the fate of cells after radiation-induced DNA damage are not well understood. This is a major clinical problem, as determines the success of radiation therapy and therefore the likelihood of developing a tumor relapse. We established a model system of intra-tumoral heterogeneity and divergent cell fate decisions by studying genetic subclones with different responses to radiation. To understand their differential behaviour in terms of signalling dynamics, we performed time-course mass cytometry (CyTOF) analyses of irradiated and non-irradiated cells. We so far uncovered plausible molecular mechanisms of resistance, that will be tested by perturbing signalling pathways.
I generally like data-driven analyses, which allows me to come up with new hypothesis that can be further tested. I’m interested in the DNA-damage response, specifically after ionising radiation, and the involvement of p53 and MAPK pathways. I enjoy single cell analyses, understanding the data by disentangling different sources of covariance like cell cycle, cell volume, and other cell state dynamics.
Finnja Becker
Supervisors: Anton Henssen

Dileshi Bulathsinhala
Discovering molecular insights for combatting therapy resistance in neuroblastoma with computational modelling
supervisors: Nils Blüthgen, Hanspeter Herzel
In the discipline of paediatric oncology, my project focuses on neuroblastoma. The overall long-term survival rate for patients with stage 4 neuroblastoma is poor, and this is primarily due to lethal relapses that result from therapy resistance and early metastasis. In order to combat therapy resistance, we require molecular insights to tumour evolution. I will apply computational modelling to analyse the impact of different RAS pathway mutations on the feedback and rewiring of the signalling pathway in order to understand resistance development. Our goal is to identify combinatorial treatments capable of sensitising highly resistant neuroblastoma cell lines.

Sinduja Chandrasekaran
Analyses of genomic rearrangement breakpoints in neuroblastoma
supervisors: Birte Kehr, Johannes Schulte

Emel Comak
Inference of cis-regulatory mutations in cancer
Supervisors: Martin Vingron, Roland Schwarz

Abeera Fatima
Network biology for precision medicine
Supervisors: Martin Vingron
Drug toxicity is a major problem for drug development. On one hand many clinical phase I studies fail because of unforeseen toxicity of the drug candidates. On the other hand, for approved drugs many therapies, for example cancer therapies, have side effects that harm patients because of toxic processes induced by the drugs. This project addresses these issues by building a workflow that utilizes extensive molecular information on drug action from a large body of publicly available omics data on human tissues. Thereby, a novel methods for the graph-based analysis of temporal gene expression data and the construction of tissue-dependent protein-protein interaction networks in order to improve network propagation methods based on random walk with restart will be developed. Results of the project will contribute to a better molecular characterization of drug toxicity pathways in various human tissues and thus to a more specified and accurate drug safety prediction in precision medicine.

Melanie Fattohi
Deciphering cell state transitions in embryo development and cancer
Supervisors: Stefanie Grosswendt, Laleh Haghverdi, Ulf Leser
My research focuses on investigating gene expression dynamics of cells in embryo development and the embryonal cancer neuroblastoma. To this end, I aim to develop computational single-cell methods for building gene regulatory networks to potentially gain new insights into normal and aberrant cell trajectories.
Jannik Franzen
Understanding Colorectal Cancer Heterogeneity: Cell deconvolution from single-cell and spatial transcriptomics
Supervisor: Dagmar Kainmüller
Angelos Galanopoulos
Supervisors: Livius Penter, Nils Blüthgen
Samuele Garda
Neural Named Entity Normalization for the biomedical domain
Supervisors: Ulf Leser, Markus Schülke
My research focuses on the automatic extraction of structured information from the scientific literature. The aim is to accelerate the integration of new knowledge into resources crucial for biomedical research, including personalized cancer treatment. Specifically, I am interested in retrieving information regarding non-coding DNA sequences and the disease potential of their variants.

Mădălina Giurgiu
Extrachromosomal circular DNA structure heterogeneity in neuroblastoma
Supervisors: Anton Henssen, Knut Reinert, and Kerstin Haase
My research focuses on characterising extrachromosomal circular DNA structure diversity in neuroblastoma. To facility the study are developing methods for reconstructing these circular structures based on long-read nanopore data.
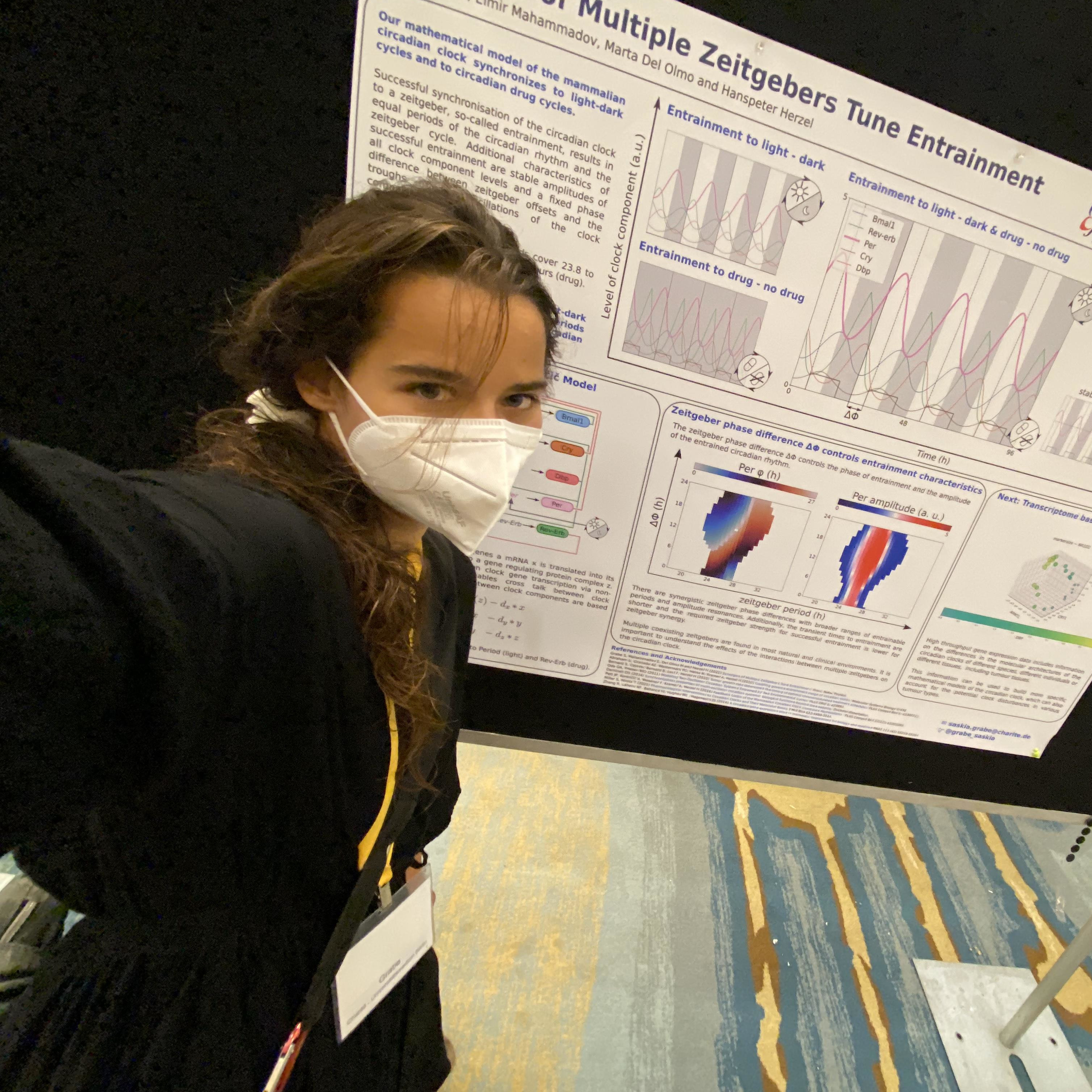
Saskia Grabe
Theoretical Analysis of Cancer Chronotherapy
Supervisors: Hanspeter Herzel, Johannes Schulte
I investigate the altered dynamics of circadian rhythms in cancer tissues and the effects of circadian schedules on cancer treatment with mathematical modelling.
Aliki Grammatikaka
Supervisors: Dieter Beule

Fatemeh Habibolahi
Molecular and functional analysis of multimodal single-cell immune profiling data in allogeneic hematopoietic stem cell transplantation and immune checkpoint therapy
Supervisors: Il-Kang Na, Dieter Beule, Benedikt Obermayer
This project aims to enhance understanding of T cell behavior in immunological treatments like allogeneic hematopoietic stem cell transplantation (alloHSCT) and immune checkpoint inhibitors, particularly in managing adverse reactions such as graft-vs-host disease (GVHD) and immune-related adverse events (irAE). The research involves single-cell sequencing of gene expression, T cell receptor (TCR) and B cell receptor (BCR) repertoires from donor-recipient pairs before and after alloHSCT, combined with computational analysis to identify molecular patterns and variations. The project also focuses on developing computational models to predict TCR antigen specificity and correlate clonal identity with gene expression, leveraging existing databases and high-throughput data. Additionally, the study will analyze antigen specificities in T cells from irAE patients, aiming to predict and experimentally validate specific TCR-peptide interactions relevant to autoimmunity or tumor responses, ultimately improving therapeutic strategies.

Viola Hollek
Impact of drugs and oncogenic mutations on signalling and phenotypes in intestinal epithelium
Supervisora: Nils Blüthgen, Matthias Selbach
Oncogenic mutations activating signalling pathways such as Wnt/β-catenin and MAPK signalling are known to perturb intestinal homeostasis and thereby drive cancer development, heterogeneity and therapy resistance. However, a comprehensive understanding of the phenotypes that arise from individual oncogenic mutations in colorectal cancer is still missing. I am interested in this genotype-phenotype relationship and am working on an approach to study the changes in signalling networks, cell fate decision, and treatment sensitivity as a result of distinct genetic alterations in patient-derived organoids. Using a virus-based oncogene library, I am going to analyse the effects of individual oncogenes on a transcriptional as well as posttranslational level to define clinically relevant oncogene-specific signatures, eventually.
Wanja Kassuhn
Identification and evaluation of novel therapeutic approaches to distinct molecular subtypes of high-grade serous ovarian cancer
Supervisors: Hagen Kulbe, Nils Blüthgen, Ulf Leser

Tom Kaufmann
Evolution and heterogeneity of copy-numbers in cancer genomes
Supervisors: Klaus-Robert Müller, Roland Schwarz
My research focuses on tumour heterogeneity and cancer evolution with a special focus on copy-number aberrations. To this end I developed MEDICC2 which reconstructs phylogenies from multi-sample copy-number data and am currently working on developing mutational signatures based on distinct copy-number changing events.

Nora Koreuber
Explainable AI for Biomedical Images
Supervisor: Dagmar Kainmüller
Susmita Mandal
Spatial Analysis of Extrachromosomal DNA in Neuroblastoma Tumors
Supervisors: Teresa Krieger, Anton Henssen

Javier Marchena Hurtado
Neighbor-based normalization for protein data in CITE-seq
Supervisors: Nils Blüthgen, Stefanie Grosswendt
CITE-seq is a method to perform RNA sequencing along with gaining quantitative information about surface proteins on a single-cell level. Nevertheless, CITE-seq data presents several sources of technical noise. There is therefore a need to normalize CITE-seq data in order to remove technical noise as much as possible. I focus on normalizing the protein data in CITE-seq. Specifically, I focus on reducing technical noise in protein data caused by different sequencing depths (similar cells occasionally have different total numbers of protein counts, due to differences in the sequencing process). Here, I propose a per-cell normalization method for CITE-seq protein data where I adjust the total protein counts of each cell, thereby reducing noise due to different sequencing depths.

Sofya Marchenko
Towards overcoming endocrine resistance in breast cancer
Supervisors: Christine Sers, Nils Blüthgen
Although endocrine therapy to block the ER pathway is highly effective and continues to be the mainstay for ER+ Breast Cancer (BC) patients, de novo resistance is common. In parallel to hormone-dependent growth in BC, tumor cells are known to heavily depend on growth factor receptor mediated signaling to execute their pathological behaviors. My aim is to gain insight into the drivers of endocrine therapy resistance using computational methods, such as machine learning and network analysis. Making use of multiple layers of omics data and integrating them with clinical data, I hope to find molecular signatures to elucidate the escape pathways, which provide tumors with alternative proliferative and survival stimuli. Due to the complexity of each patient case, understanding the activated pathways in endocrine resistant patients will help to identify those patients whose tumors are most likely to benefit from specific cotargeting strategies, which is an important step towards personalized therapy in BC.
Twitter: @SofyaMa

Vinzenz May
Long read based structural variants
Supervisors: Dieter Beule
Genomic structural variants (SVs) are responsible for a wide range of rare diseases and play an important role in many cancers. A structural variant is a re-arrangement of DNA in a chromosome, e.g. duplication of a larger region or translocation from one chromosome to another. They can have direct or regulatory influence on the expression of genes and splicing variants. SVs are still difficult to correctly detect and genotype because of the limited span of basepairs per read. Such reads are generated with widely used technologies such as short read sequencing (< 300 bp). Longer reads (> 10kb) have been much more error-prone in the past but become similarly precise as short reads in resolving each single base correctly. To facilitate a broad usage of long read sequencing in clinical diagnostics, we still need to develop software to correctly find and genotype SVs in families or groups of patients or sub-clones of cancer tumors. My Project aims to develop a new pipeline and new algorithms to find all SVs in family genomes or tumor genomes so that researchers have a better and reliable tool at their hands to learn about the roles of SVs in many rare diseases and cancer types.

Fabian Reith
Tackling cancer pathologies via the application of artificial intelligence, specifically deep learning
Supervisor: Dagmar Kainmüller, Christine Sers
Tackling cancer pathologies via the application of artificial intelligence, specifically deep learning, under the supervision of Dagmar Kainmüller and Christine Sers. My work focuses on PD-L1 prediction in Angiosarcoma and other rare cancers based on IHC stained whole slide images (WSIs). There, I developed a tool to determine PD-L1 tumor proportion scores with the aim to augment pathologist decision making. Additionally, I am exploring unsupervised domain adaptation techniques to enhance cell segmentation performance on out-of-domain data.

Lorenz Rumberger
Deep Learning for Microscopy Image Analysis
Supervisors: Dagmar Kainmüller, Markus Morkel
Spatial biology, the study of cells and their interactions in context of their tissue microenvironment, heavily relies on automated microscopy image analysis to segment cells and quantify local characteristics of tissues. My PhD focuses around method development to further improve the image analysis step and on analyzing microenvironments and local cell-type enrichment in colorectal cancer.

Jennifer von Schlichting
Modeling the Tumor Microenvironment in Patient-Derived Organoid Culture
Supervisors: Nils Blüthgen, Chris Sander, Markus Morkel
Patient-derived organoids (PDOs) are a model of choice to elucidate inter- and intratumoral heterogeneity to combat therapy resistance. However, the utility of PDOs is limited by heterologous and poorly-defined extracellular matrices and lack of proper tumor microenvironment, thus failing to model the tumor in its complexity. I am working on developing an approach to identify relevant paracrine interactions between stromal and tumor cells in colorectal cancer (CRC). Single cell-RNAseq data of 12 CRC patients were analyzed for ligand-receptor pairs enabling stroma-to-tumor signaling. Further, I aim to model ECM composition in colorectal cancer, by supplementing the laminin/collagen IV rich environment with other known ECM proteins such as collagen I to identify the impact of a changing substrate on cell plasticity.
In an appropriate in vitro assay, I assess physiological relevance based on single cell analysis. Thus, I am trying to identify paracrine factors and signals affecting proliferation, differentiation, and developmental trajectories of CRC PDOs in vitro. I hypothesize that environmental factors may limit the phenotypic space in which organoid cells differentiate, disabling the study of more invasive behaviors in vitro. Recent data show that ECM parameters have a strong impact on cell plasticity and highlight the importance of adjusting and expanding organoid in vitro culture models. It is my goal to provide guidelines to improve existing PDO CRC models and provide a feasible approach to address common limitations in organoid culture. Ultimately, I am interested in identifying factors that can interfere with drug efficacy and potentially favor clinically relevant therapy resistance mechanisms.
Katharina Schneider
Supervisors: Nils Blüthgen
Oğuz Serbetci
Supervisors: Ulf Leser

David Steinbrecht
Modelling the influence of signalling on transcript dynamics
Supervisors: Nils Blüthgen, Markus Landthaler
Activation of cellular signalling networks influences a cell’s transcriptional output which is closely linked to its biological function and state. Mutations in signalling pathways are associated with the pathogenesis and progression of different cancer types. I’m interested in how specific pathways influence transcription and consequently cell behaviour. On the basis of time-resolved RNA sequencing data I try to gain insight into the kinetics of fundamental processes that control gene expression using computational modelling. My aim is to provide values of key parameters in these regulatory system that others can rely on and use in higher-level cancer research.

Maja-Celine Stöber
Linking transcriptomic and genomic tumour heterogeneity in neuroblastoma
Supervisor: Roland Schwarz, Anton Henssen, Jan Philipp Junker

Karina Tristan
Generating a colorectal cancer specific oncogenic stress signature by integrating transcriptomic(bulk/single-cell) and proteomic datasets (DRAFT)
Supervisors: Christine Sers, Nils Blüthgen
Colorectal Cancer(CRC) is one of the main causes of death by cancer worldwide. It is a highly heterogeneous cancer that can be caused by several mutations the more known to be KRAS and BRAF. BRAFmut cancer is recognized as the most aggressive and has a tendency of high recurrence after treatment. Oncogenic stress signatures aimed for the classification and recognition of BRAFmut CRC are still unknown. Several problems arise to address the question. We first need to determine what is the "type" of stress and at which level(RNA/protein) are to be discussed, such as replication stress or oxidative stress. Secondly, is this stress signature able to determine the sensitivity of the cells? For these questions, a compilation of stress signatures is to be collected and applied on RNAseq data to generate a new signature specific for CRC BRAFmut, this signature then will be tried out as a biomarker after gene scoring is done. After the signature is generated it needs to be tested to check whether a correlation or difference is seen by cellular phenotype, cell/tissue types and cell states. This is to be approached by the analysis of single-cell data. Preliminary analysis with a "lab-made" signature has been done in publicly available data with rather inconclusive results, the use of other signatures for comparison is to be done as well as the use of different datasets. Gene scoring methods are being analyzed for the different signatures collected for further analysis.

Xing Wang
Question Answering for Precision Oncology
Supervisors: Ulf Leser, Il-Kang Na
Personalized therapies become increasingly available for cancer treatment and offer important alternatives especially where standard therapies fail. For a specific genomic profile of a patient, often, an extensive literature research has to be conducted to find a possible treatment. In this project, we want to facilitate the literature review process by employing text mining and machine learning methods to gather the desired information automatically from the biomedical literature. The model is designed to directly answer questions posed by clinicians and provide the corresponding literature references with it.

Tzu-Ting Wei
Analysing tumour heterogeneity in single-cell transcriptomics using somatic variants
Supervisors: Dieter Beule, Nils Bluethgen, Christine Sers
Single-cell techniques enable detailed characterisation of tumour heterogeneity. Our recent study suggested that normal cells adjacent to tumour cells exhibit a more stem-like phenotype, possibly triggered by the tumour microenvironment. However, reliable identification of tumour cells is often challenging. It is feasible to differentiate tumour cells from normal cells in copy number altered tumours via copy number variation (CNV) inference methods from transcriptomes. In contrast, copy number-based methods can not work with tumours which exhibit more single nucleotide variants (SNV) but few copy number changes. Somatic variant calling methods developed from bulk RNA sequencing data have been applied to single-cell data, while all methods suffer from the precision and recall trade-off. In this project, I will use whole-genome and whole-exome genomics data and single-cell transcriptomes to investigate the non-random distribution of tumour-associated SNVs.
Follow me on Twitter @TzuTingCindy
Robin Xu
Mutational landscape on extrachromosomal circular DNA (ecDNA)
Supervisors: Anton G. Henssen,Leif S. Ludwig,Frederik Damm,Kerstin Haase

Erika Zuljan
Comprehensive analysis of the tumor microenvironment in advanced salivary gland cancers
Supervisors: Dieter Beule, Damian Tobias Rieke, Stefanie Großwendt
Salivary gland cancers (SGC) are rare malignancies that arise from the tissue of salivary glands and account for 5% of all head and neck cancers. The second most common histology is ACC (Adenoid Cystic Carcinoma), with a very poor outcome due to the lack of established therapy options. Compared to other entities, ACC is defined as immune-deserted. Exome (WES), genome (WGS) and bulk RNA-seq from patients with advanced ACC and other entities were analyzed to gain insights in the mutational landscape, transcriptome and tumor immune microenvironment (TIME). The cohort of 97 patients is one of the largest cohorts in the world for advanced salivary gland cancer. Preliminary result show a lower immune-infiltration in most of the ACC tumors, with the exception of small subset. Single cell data is being produced and analyzed to characterize the immune-activated ACCs and the TIME of ACCs compared to other entities. The aim here is to find potential targets for therapy for advanced ACC,with a focus on immuno-therapy.